Экологичность продукции Уралпротект
Каждый современный производитель обязан отвечать за здоровье персонала своего предприятия и персонала предприятий заказчиков, работающих с его продукцией. Кроме того, осознавая текущие проблемы, связанные с загрязнением нашей среды обитания, на производствах должны соблюдаться принципы заботы об окружающей среде, минимизации выбросов вредных веществ в атмосферу и сточные воды, принцип безотходного производства и рекуперации энергии, тепла и т.д. Наша компания стремиться следовать всем вышеуказанным принципам, наши продукты не содержат запрещенных в производстве ЛКМ, вредных для здоровья персонала и конечного пользователя веществ; наше производство организовано таким образом, чтобы оптимизировать расход сырья, в частности органических растворителей, есть возможность их восстановления и очистки.
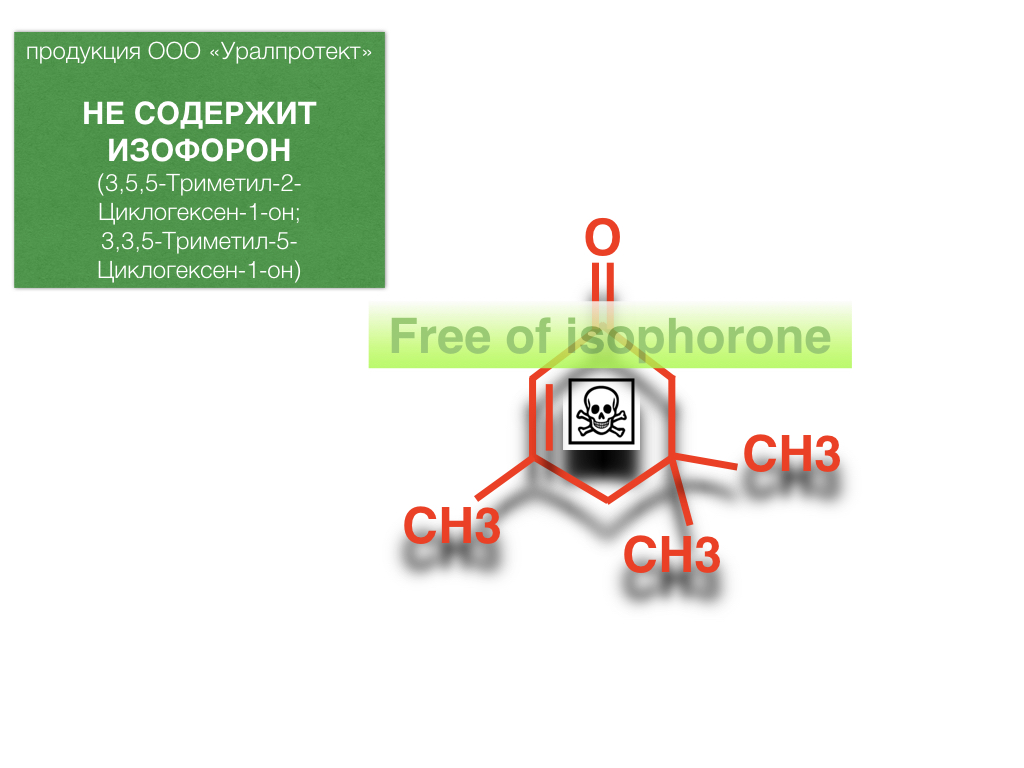
Мы не используем изофорон при производстве наших продуктов, поскольку осознаем какое негативное токсическое и канцерогенное воздействие он оказывает на организм человека.
Изофорон - ненасыщенный циклический кетон, органический растворитель, смешивающийся в любых пропорциях с другими органическими растворителями.Он является одним из наиболее эффективных полярных растворителей, растворяет многие природные и синтетические смолы и полимеры, такие как: поливинилхлорид и сополимеры винилхлорида, поливинилацетат, полиакрилаты, полиметакрилаты, полистирол, хлоркаучук, алкидные смолы, насыщенные и ненасыщенные полиэфиры, эпоксидные смолы, нитроцеллюлоза, сложные и простые эфиры целлюлозы, даммаровая смола (депарафинизированная), каури, воски, жиры, масла, фенол-, меламин- и карбамидоформальдегидные смолы. Изофорон уменьшает вязкость и способствует улучшению адгезии за счет набухания подложки, именно это свойство делает его популярным в химической промышленности.
Минусами данного растворителя являются в первую очередь токсическое (наркотическое) и канцерогенное воздействие на организм человека, опасен как физический контакт изофорона с кожными покровами и слизистыми оболочками человека, так и попадание его паров в легкие при вдыхании.Применение данного растворителя в производстве ЛКМ запрещено различными директивами в странах Европейского союза и США, т.к. доказано, что изофорон может вызывать рак, оказывает негативное влияние на эндокринную систему, слизистые оболочки глаз, кожу и легкие. Предельно допустимая концентрация (ПДК) паров изофорона в атмосфере рабочего помещения составляет всего 1 мг/м3.
Не смотря на то что изофорон в настоящий момент не запрещен к применению в лакокрасочной отрасли на территории РФ, мы категорически против использования его в нашей продукции. Здоровье и безопасность наших сотрудников стоит для ООО «Уралпротект» на первом месте, т.к. залогом успешной работы и высокой производительности труда является здоровый персонал и руководство. Такой же позиции мы придерживаемся относительно наших заказчиков. На завод заказчика не должно поставляться сырье и продукты, содержащие в себе потенциально опасные для здоровья персонала компоненты!
Мы не используем в нашем производстве сырье, в состав которого входят запрещенные химические соединения, содержащие шестивалентный хром, Cr (VI).
Огромную опасность для окружающей среды наряду с нефтью представляют тяжелые металлы: ртуть, свинец, кадмий, медь, цинк, хром.Тяжелые металлы, попадая в водоемы со сточными водами промышленных предприятий, вызывают необратимые изменения природных экосистем. Для тяжелых металлов в природе не существует механизмов самоочищения, как это свойственно органическим соединениям, они лишь перемещаются из одного природного резервуара в другой. Влияние восьми металлов (в т.ч. и хрома) выделено Агентством по охране окружающей среды как приоритетное.
Среди группы тяжелых металлов важное значение придается соединениям хрома. Загрязнение окружающей среды соединениями хрома может быть потенциально опасно для человека и других биологических видов. Количество соединений хрома в окружающей среде возрастает с каждым годом и достигает уровня, опасного для жизнедеятельности организмов.
Химические соединения хрома, в которых данный элемент находится в шестивалентной форме, Cr(VI), являются наиболее опасными для человека и окружающей среды. Шестивалентный хром входит в состав коррозионностойких, защитных конверсионных хроматных покрытий (плёнок), которые наносят на многочисленные ответственные детали транспортных средств, таких, как крепежные детали, кронштейны, скобы, рычаги, элементы сцепления, подвески и т.д. Он также может быть обнаружен в полимерных пигментах, чернилах, в нержавеющей стали (Cr6+ выделяется в ходе литья, плавления, факельной резки), текстильных красителях (хроматы натрия и калия и бихроматы аммония и калия). Хроматы цинка и свинца являются генотоксичными канцерогенными веществами. Хроническое вдыхание соединений шестивалентного хрома увеличивает риск заболеваний носоглотки, риск рака лёгких. (Лёгкие особенно уязвимы из-за большого количества тонких капилляров).

Многие государственные стандарты и директивы не допускают использование шестивалентного хрома в органорастворимых эмалях, так, например, в ГОСТ 33290-2015 «Материалы лакокрасочные, применяемые в строительстве. Общие технические условия» в перечне тяжелых металлов, запрещенных к применению в ЛКМ для внутренних работ, наряду со свинцом указан шестивалентный хром.
Наиболее широко шестивалентный хром применяется в качестве коррозионно-защитного хроматного слоя на оцинкованных стальных поверхностях. Это влажные, студенистые плёнки, высыхающие на поверхности. После механического повреждения, нарушения сплошности, появления трещин плёнки проявляют способность к самозалечиванию, самовосстановлению, что делает их очень эффективными коррозионно-защитными покрытиями.
В настоящее время из-за вступления в действие директив, ограничивающих применение шестивалентного хрома, наиболее остро встает проблема его замены. Было разработано большое количество вариантов для замены шестивалентного хрома, включающих толстые цинк–органические покрытия, цинковые порошковые сплавы, составы на основе трёхвалентного хрома и т.д. Коммерчески приемлемыми являются несколько заменителей на основе трёхвалентного хрома.



